
FDA Warns About Fake Botox. The U.S. Food and Drug Administration (FDA) is warning consumers to be aware of fake botox. In a safety notification posted on the agency’s website on April 16th, the FDA said a counterfeit version of Botox was identified in the United States. The bogus products may have been sold […]
FDA Warns About Fake Botox. The U.S. Food and Drug Administration (FDA) is warning consumers to be aware of fake botox. In a safety notification posted on the agency’s website on April 16th, the FDA said a counterfeit version of Botox was identified in the United States. The bogus products may have been sold to doctors’ offices and medical clinics across the country. The FDA says the counterfeit product was sold by an unlicensed supplier who is unauthorized to distribute drug products in the US.
“The counterfeit products are considered unsafe and should not be used. FDA cannot confirm that the manufacture, quality, storage, and handling of these suspect products follow U.S. standards.” the FDA stated in the notification. Botox is used to treat wrinkles, headaches, severe underarm sweating and other conditions.
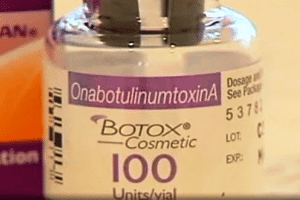
FDA-approved Botox products are manufactured by Allergan and lists “OnabotulinumtoxinA” as the active ingredient on the outer carton and vial. The fake and FDA-approved products have some similarities. Healthcare professionals and consumers can identify fake botox products by looking out for a missing lot number on the vial, blank entries next to the LOT: MFG: EXP:, and “Botulinum Toxin Type A” instead of “OnabotulinumtoxinA” as the active ingredient.
The personal injury attorneys at Parker Waichman LLP offer free, no-obligation case evaluations. For more information, fill out our online contact form or call 1-800-YOURLAWYER (1-800-968-7529).
Our Botox side effects attorney is here to help you when you need it the most.


