
Manufacturers have been linked to a painful and debilitating condition called Post Arthroscopy Glenohumeral Chondrolysis. Pain pmps made I-Flow, Stryker, Sorenson and other manufacturers have been linked to a painful and debilitating condition called Post Arthroscopy Glenohumeral Chondrolysis (PAGCL). If you underwent shoulder surgery that involved the implantation of a I-Flow, Stryker, or Sorenson pain pump, and […]
Manufacturers have been linked to a painful and debilitating condition called Post Arthroscopy Glenohumeral Chondrolysis. Pain pmps made I-Flow, Stryker, Sorenson and other manufacturers have been linked to a painful and debilitating condition called Post Arthroscopy Glenohumeral Chondrolysis (PAGCL). If you underwent shoulder surgery that involved the implantation of a I-Flow, Stryker, or Sorenson pain pump, and were diagnosed with this condition, you may be entitled to compensation for your injuries.
Dozens of lawsuits have been filed against I-Flow, Stryker, Sorenson by the victims of shoulder pain pump injuries. These lawsuits claim the manufactures of pain pump knew they were dangerous when used in joint surgery, and that they ignored the finding from various studies. The manufacturers of pain pumps also stand accused of promoting unapproved uses of the products.
Earlier this month, an Oregon jury ordered I-Flow to pay $5.5 million to a Portland man and his wife after finding the company liable for damaged cartilage to the man’s right shoulder. The lawsuit alleged that I-Flow knowingly marketed its On-Q Painbuster to orthopedic surgeons even though the FDA rejected approving the device for that use. According to the Oregon lawsuit, despite three rejections from the Food & Drug Administration, I-Flow issued a press release on June 2, 1998, announcing that the agency had approved its pain pump for orthopedic use.
approved by the FDA
According to court documents, the plaintiff’s doctor had no idea that the I-Flow pain pump he used was not approved by the FDA, nor did he know the pump actually caused the permanent damage to his patient’s shoulder joint. The man’s doctor would later discover that nearly 49 patients he had treated with the I-Flow pain pump had similar complications after surgery.
PAGCL is a relatively rare condition in which joint cartilage in the shoulder dies following the use of I-Flow, Stryker, Sorenson and other brands of pain pumps. PAGCL can cause a lifetime of pain and disability, and in the most severe cases, victims have had to undergo joint replacement surgery.
Symptoms of PAGCL include:
• Shoulder Pain (in motion or at rest)
• Shoulder Stiffness
• Clicking/Popping Shoulder
• Grinding Shoulder
• Shoulder Weakness
• Inhibited Shoulder Motion
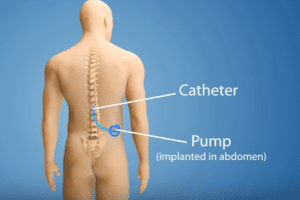
Commonly called intra-articular pain pumps, devices made by I-Flow, Stryker, Sorenson and are used after surgeries to deliver local anesthetics to a specific area through a plastic tube. Such pain pumps became popular with orthopedic surgeons in the late 1990s because they were seen as an alternative to extended hospital stays and safer than prescribing narcotic painkillers. However, they were never actually approved by the Food & Drug Administration (FDA) for use in joint surgery.
According to The New York Times, in 1998, one pain pump manufacturer, McKinley Medical, asked the FDA for permission to revise its labeling to indicate that the pumps could be inserted in joints. The agency refused because it said no previous devices had proved such a use to be safe. Despite the FDA’s stance, such devices were increasingly used off-label in joint surgeries.
When used in shoulder surgery, the intra-articular pain pump catheter is placed into the shoulder joint. The pump remains in the shoulder for several days to deliver controlled doses of pain medication. T. Unfortunately, use of pain pumps in this way carries serious risks. Exposing cartilage to the painkiller for such an extended amount of time has a toxic effect. In 2007, the American Journal of Sports Medicine showed a direct link between PAGCL and the use of pain pumps during shoulder surgery. Other studies have produced similar findings.
In November 2009, the FDA issued a warning about the association between chondrolysis and pain pumps. At the time, the agency said it had reviewed 35 reports of the condition in patients given continuous intra-articular infusions of local anesthetics with pain pump devices.
Almost all of the reported cases of chondrolysis (97%) occurred following shoulder surgeries, the FDA said. At the time the FDA issued the warning, it ordered manufacturers of local anesthetics and pumps to change labels to discourage doctors from such uses.
The personal injury attorneys at Parker Waichman LLP offer free, no-obligation case evaluations. For more information, fill out our online contact form or call 1-800-YOURLAWYER (1-800-968-7529).


