
Daypro Side Effects Injury Lawsuits. Daypro is a prescription Nonsteroidal Anti-Inflammatory (NSAID) drug manufactured by G. D. Searle and Company, used to treat the pain and inflammation of osteoarthritis and rheumatoid arthritis. The FDA approved Daypro in 1992, after it had rejected the drug’s approval on three previous submissions. The application for approval took approximately […]
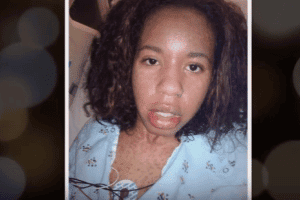
Daypro Side Effects Injury Lawsuits. Daypro is a prescription Nonsteroidal Anti-Inflammatory (NSAID) drug manufactured by G. D. Searle and Company, used to treat the pain and inflammation of osteoarthritis and rheumatoid arthritis. The FDA approved Daypro in 1992, after it had rejected the drug’s approval on three previous submissions. The application for approval took approximately 10 years because there was not substantial evidence that it was any more effective than existing drugs to treat arthritis and because of increased incidence of certain adverse events.
Daypro has an extremely long half-life (time it takes for the blood plasma concentration of the drug to be reduced by one-half), which means that the amount of drug available for therapeutic and toxic effects is greater in the blood stream for a longer period of time. The long half-life increases Daypro’s propensity to cause toxic reactions, particularly in women and persons of low body weight.
In its first year on the market, Daypro had a higher reported rate of Stevens Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (TEN) per million prescriptions than any other prescription NSAID in its first marketing year in the U.S. Stevens Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (TEN) are two forms of the skin disease that can cause rash, skin peeling, and sores on the mucous membranes. By April 1994, four deaths had been caused by serious skin reactions associated with Daypro. Daypro caused more deaths from serious skin reactions, including SJS and TEN, between 1993 and 1997 than any other prescription NSAID.
If you or a loved one took Daypro and suffered side effects, please fill out the form at the right for a free case evaluation by a qualified drug side effects attorney or call us at 1-800-YOURLAWYER (1-800-968-7529).


