
Stroke A Leading Cause Of Death. A stroke or (brain attack) takes place when a blood clot blocks a blood vessel or artery. Additionally a stroke can happen when a blood vessel breaks, disrupting blood flow to an area of the brain. When a brain attack occurs, brain cells are destroyed immediately in the area. […]
Stroke A Leading Cause Of Death. A stroke or (brain attack) takes place when a blood clot blocks a blood vessel or artery. Additionally a stroke can happen when a blood vessel breaks, disrupting blood flow to an area of the brain. When a brain attack occurs, brain cells are destroyed immediately in the area. These cells usually die within minutes to a few hours after a stroke starts. Strokes are the nation’s third leading cause of death, behind Heart Disease and Cancer. Approximately 700,000 Americans each year suffer a new or recurrent stroke.A stroke takes place nearly every 45 seconds. Strokes kill an estimated 163,000 people a year. Just about every 3 minutes someone dies from a stroke. Of every 5 deaths from a stroke, 3 occur in women and 2 in men. The 2002 stroke death rates per 100,000 populations for specific groups were 54 for white males, 53 for white females, 82 for African American males and 72 for African American females.
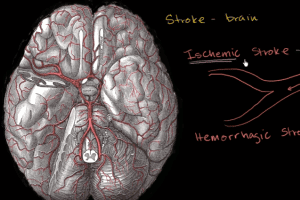
Ischemic strokes account for close to 83% of all cases. Ischemic ‘strokes’ form as a result of an obstruction within a blood vessel supplying blood to the brain. The main condition for this type of obstruction is the development of fatty deposits lining the vessel walls. This condition is called atherosclerosis. These fatty deposits can cause two types of obstruction: cerebral thrombosis and cerebral embolism.
Cerebral thrombosis refers to a thrombus (blood clot) that develops at the clogged part of the vessel. Cerebral embolism refers to a blood clot that forms at another location in the circulatory system, usually the heart and large arteries of the neck and upper chest. Another major cause of embolism is an irregular heartbeat, known as atrial fibrillation. Conditions where clots can form in the heart, dislodge and travel to the brain are created.
Hemorrhagic strokes represent about 17% of stroke cases. Hemorrhagic strokes result from a weakened vessel that ruptures and bleeds into the surrounding brain. The blood accumulates and compresses the surrounding brain tissue. There are two types of hemorrhagic strokes: intracerebral hemorrhage and subarachnoid hemorrhage.
Hemorrhagic stroke occurs when a weakened blood vessel ruptures. Two types of weakened blood vessels usually cause hemorrhagic strokes: aneurysms and arteriovenous malformations (AVMs). An aneurysm is a ballooning of a weakened region of a blood vessel. If left untreated, the aneurysm continues to weaken until it ruptures and bleeds into the brain.
An arteriovenous malformation (AVM) is a cluster of abnormally formed blood vessels. Any one of these vessels can rupture, also causing bleeding into the brain. Transient ischemic attacks – Also called TIAs, transient ischemic attacks are minor or warning strokes. In a TIA, conditions indicative of an ischemic stroke are present and the typical stroke warning signs develop. However, the obstruction (blood clot) occurs for a short time and tends to resolve itself through normal mechanisms.
Symptoms of Strokes include: confusion, trouble speaking or understanding, trouble seeing in one or both eyes, numbness or weakness of face, arm or leg, especially on one side of the body, trouble walking, dizziness, loss of balance or coordination, severe headache with no known cause, vomiting, nausea, fever, fainting, coma and convulsions.
Another major contributing factor causing strokes in the use of prescription medcines. The below is a list of the drugs associated with stroke:
If you or a loved one has taken Atypical Antipsychotics, ADHD drugs, HRT drugs or COX-II Inhibitors and suffered a stroke, please fill out the form at the right for a free case evaluation by a qualified defective drug attorney or call us at 1-800-YOURLAWYER (1-800-968-7529).


