
The Thoratec HeartMate II LVAS has been recalled in what the U.S. Food and Drug Administration (FDA) has deemed a Class I recall. This designation is the agency’s most serious and is reserved for those situations in which there exists a reasonable probability that the use of or exposure to the recalled product—in this case, […]
The Thoratec HeartMate II LVAS has been recalled in what the U.S. Food and Drug Administration (FDA) has deemed a Class I recall. This designation is the agency’s most serious and is reserved for those situations in which there exists a reasonable probability that the use of or exposure to the recalled product—in this case, the Thoratec HeartMate II LVAS—will cause serious adverse health consequences or death.
If you or a loved one suffered injuries from a Thoratec HeartMate II LVAS, please fill out the form at the right for a free case evaluation by a qualified defective medical device attorney or call us at 1-800-YOURLAWYER (1-800-968-7529).
Thoratec initiated the recall over a blood flow problem that involves a defect in which the device’s bend relief detaches from its intended position around the proximal outflow graft. The defect may allow the graft to kink or deform, which can lead to a reduction of blood flow from the HeartMate II LVAS pump, pump/graft thrombosis, or perforation of the outflow graft. The metal end of the bend relief may also be sharp and may cause erosion and cutting of the outflow graft. Thoratec HeartMate II LVAS model numbers 103393, 103695, 104692, 104911, 104912 are involved.
The recall does not involve return of the device, but rather a change in the labeling on the Thoratec HeartMate II LVAS to provide instructions on how to verify if the bend relief is fully engaged with the sealed outflow graft at the time of implant. Documentation has been revised to include a new caution statement regarding the bend relief connection and clinicians have been advised to follow the revised instructions that clarify the recommended procedure for securing the bend relief to the outflow graft. The Wall Street Journal explained that patients with the recalled HeartMate II may continue to use the device; however, future implantations should follow the new warnings and instructions.
The term “recall” is used “when a medical device is defective, when it could be a risk to health, or when it is defective” and also a health risk. The term does not always mean that patients must cease using the device, according to the FDA.
Meanwhile, The Journal pointed out that the most recent FDA announcement is actually a repost of a prior FDA decision to assign its most serious recall classification to the Thoratec HeartMate II. The decision initially appeared on the FDA web site on March 23. A month prior, on February 23, Thoratec first advised physicians of the problem and the labeling revisions. The notification indicated that if the pump was not properly connected, low pump flow or worsening heart failure symptoms could occur.
Prior to physician notification, Thoratec received 29 reports of the heart pump not being properly connected. In five cases, patients needed surgery; in one case, a patient died, although the cause was unclear, said Thoratec, according to The Journal piece.
The Thoratec HeartMate II LVAS is a heart pump device used for what the FDA described as a bridge to transplantation in heart transplant candidates at risk of imminent death from nonreversible left ventricular failure. The $100,000 system is also used in patients with New York Heart Association (NYHA) Class IIB of IV end-stage left-ventricular failure who have received optimal medical therapy for at least 45 of the prior 60 days, but who are not heart transplant candidates.
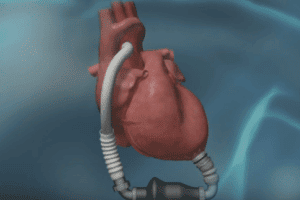
The Thoratec HeartMate enables blood delivery from the dysfunctional left ventricle of the patient’s heart to the body and is an axial-flow, rotary ventricular assist system that can generate blood flows up to 10 liters per minute. Meant for home and hospital use, the Thoratec HeartMate is also used during transport of ventricular assist device patients by ground ambulance, airplane, or helicopter.
The system includes an implanted pump connected by a cable to an external controller and batteries; the device is implanted in the abdomen and a connector—the inflow conduit—moves blood from the heart into the pump. Another tube—the outflow tube, or graft—brings blood from the pump to the aorta, Reuters recently explained.
An earlier version of the device, the bend relief and outflow graft were attached to the pump; however, early last year, Thoratec released the new version with a detachable bend release with the intention that the enhancement would enable surgeons to more easily remove air from the graft, according to a Reuters news release on the problem.
There is no alternate therapy to HeartMate for the heart-failure patient population.
At year-end 2011, over 4,000 HeartMate II patients were receiving ongoing life-restoring support, said Thoratec, which noted that former Vice President Dick Cheney utilized a HeartMate device until his recent heart transplant.
The personal injury attorneys at Parker Waichman LLP offer free, no-obligation case evaluations. For more information, fill out our online contact form or call 1-800-YOURLAWYER (1-800-968-7529).


