Medtronic Insulin Pump Recall Initiated In Light of Cybersecurity Risks
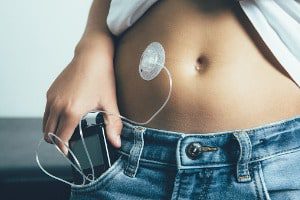
Medtronic has recently issued a recall of certain products in the MiniMed insulin pump product line. The Medtronic insulin pump recall began after the U.S. Food and Drug Administration (FDA) [...]