
Bisphosphonate Labels With Fracture Warning. Health Canada has announced that its review of bisphosphonates has shown a slightly increased risk of atypical thigh fractures among people who use these medications. Bisphosphonates, a class of drugs used to treat osteoporosis, are sold in Canada under the brand names Fosamax, Fosavance, Didrocal, Actonel and Aclasta. In the U.S., brand […]
 Bisphosphonate Labels With Fracture Warning. Health Canada has announced that its review of bisphosphonates has shown a slightly increased risk of atypical thigh fractures among people who use these medications.
Bisphosphonate Labels With Fracture Warning. Health Canada has announced that its review of bisphosphonates has shown a slightly increased risk of atypical thigh fractures among people who use these medications.
Bisphosphonates, a class of drugs used to treat osteoporosis, are sold in Canada under the brand names Fosamax, Fosavance, Didrocal, Actonel and Aclasta. In the U.S., brand names bisphosphonates include Actonel, Aredia, Bonefos, Boniva, Fosamax, Didronel, Reclast, Skelid and Zometa.
Canadian labels for or brand name bisphosphonate drugs have been updated with new warnings and precautions regarding their association with atypical thigh fractures, according to Health Canada The new information includes signs of a possible atypical femur fractures that patients and health professionals should watch for. Labeling for generic bisphosphonates will be updated with this new information in the near future.
According to Health Canada, atypical femur fractures are rare, accounting for less than 1% of all hip and femur fractures overall.
While the agency’s review of the evidence has shown a slightly increased risk of this type of fracture with bisphosphonate use, Health Canada said that benefits of using bisphosphonate drugs in preventing broken bones from osteoporosis may outweigh the risk sustaining an atypical femur fracture.
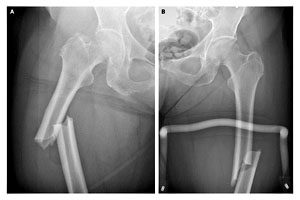
An atypical femur fracture can occur with minimal or no impact to the thigh area, and can occur in both legs in the same person. Symptoms of these types of fractures include dull, aching pain in the thigh, hip or groin area. A partial fracture could take weeks or months to become a complete fracture.
Health Canada is advising patients currently taking or who have taken a bisphosphonate drug in the past, and who notice any of these symptoms, to seek medical attention immediately, as they may be a sign of an atypical femur fracture. However, patients should not stop taking their bisphosphonate drug unless advised to do so by their healthcare professional, the agency said.
Health Canada has also advised doctors to be aware of the possible risk of atypical femur fractures in patients taking bisphosphonates.
They should evaluate patients who report new hip, thigh or groin pain to rule out a partial femur fracture. Patients with an atypical femur fracture should also be assessed for possible signs of fracture in the other leg.
Discontinuation of bisphosphonate therapy should be considered pending an assessment of the patient or the risk/benefit of using it. Health Canada also reminded doctors that the need for continued bisphosphonate therapy should be periodically re-evaluated.
The personal injury attorneys at Parker Waichman LLP offer free, no-obligation case evaluations.
For more information, fill out our online contact form or call 1-800-YOURLAWYER (1-800-968-7529).


