
A Tracheostomy is a Medical Procedure. The U.S. Food and Drug Administration (FDA) has placed its highest-risk Class I label on a recall of Willy Rusch TracheoFlex Tracheostomy Tube Set. The recall is being issued because the connector can disconnect from the tracheostomy tube, which provides an airway, during ventilation. If this occurs, patients may not […]
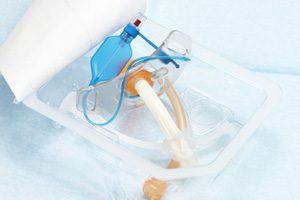
A Tracheostomy is a Medical Procedure. The U.S. Food and Drug Administration (FDA) has placed its highest-risk Class I label on a recall of Willy Rusch TracheoFlex Tracheostomy Tube Set. The recall is being issued because the connector can disconnect from the tracheostomy tube, which provides an airway, during ventilation. If this occurs, patients may not get proper ventilation, leading to serious injury or death.
A tracheostomy is a procedure where a tube is placed into the windpipe (trachea) in order to provide an airway. It is usually performed when there is an obstruction to breathing. A recall alert posted on the FDA website states, “If the connector detaches from the tracheostomy tube shaft during use, it can deprive the patient of adequate ventilation and would require immediate medical intervention including changing the tracheostomy tube and placing a new tube. The use of affected products may cause serious adverse health consequences including oxygen deprivation, brain damage and death.”
Customers were notified of the recall through an Aug. 26, 2016 “Urgent Field Safety Notice.”
Class I recalls are the most serious type of recall. The Class I designation means that exposure to a recalled device presents a reasonable risk of serious injury or death.
The recall affects certain lot numbers and product codes that were manufactured between June and December of 2015 and distributed between July 2015 and May 2016. In the United States, a total of 5 units were distributed, according to the alert.
The personal injury attorneys at Parker Waichman LLP offer free, no-obligation case evaluations. For more information, fill out our online contact form or call 1-800-YOURLAWYER (1-800-968-7529).


