
Resume Sales of Cognis, Teligin Defibrillators. Boston Scientific says its Cognis and Teligen defibrillators will be back on sale within 24 hours. Those devices were among the defibrillators recalled by Boston Scientific last month. According to The Wall Street Journal, the Food & Drug Administration (FDA) has approved two manufacturing changes that the company had […]
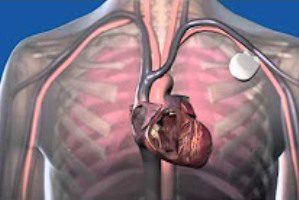
Resume Sales of Cognis, Teligin Defibrillators. Boston Scientific says its Cognis and Teligen defibrillators will be back on sale within 24 hours. Those devices were among the defibrillators recalled by Boston Scientific last month.
According to The Wall Street Journal, the Food & Drug Administration (FDA) has approved two manufacturing changes that the company had not cleared with the agency when they were implemented.
Companies are required to submit a notification of any change in manufacturing procedures or methods of manufacturing that affect the safety or effectiveness of the devices, thus last month’s recall.
The resume in the sale of Teligin Defibrillators is considered a welcome development.
The recall impacted seven brands of Boston Scientific defibrillators: COGNIS®, CONFIENT™, LIVIAN™, PRIZM™, RENEWAL®, TELIGEN® and VITALITY™
However, five older devices are still on hold, following disclosure by the company that it found some other manufacturing changes that should have been cleared by the FDA. It will be about three to four weeks before sales of those implants resume.
The personal injury attorneys at Parker Waichman offer free, no-obligation case evaluations. For more information, fill out our online form or call 1-800-YOURLAWYER (1-800-968-7529).


