
DePuy ASR hip implant caused painful symptoms. Parker Waichman LLP, a national law firm dedicated to protecting the rights of victims injured by defective medical devices, has filed a lawsuit alleging that the DePuy ASR hip implant caused painful symptoms in a Seminole, Florida resident. The suit was filed on August 24th in the U.S. District […]
DePuy ASR hip implant caused painful symptoms. Parker Waichman LLP, a national law firm dedicated to protecting the rights of victims injured by defective medical devices, has filed a lawsuit alleging that the DePuy ASR hip implant caused painful symptoms in a Seminole, Florida resident. The suit was filed on August 24th in the U.S. District Court for the Northern District of Ohio, Western Division (Case No. 1-12-dp-23381).
There, it is one of the many cases that have been filed into the multidistrict litigation as part of the In Re: DePuy Orthopaedics, Inc. ASR Hip Implant Products Liability Litigation (MDL No. 1:10 md 2197). DePuy Orthopaedics, Inc., DePuy Inc., DePuy International Limited, Johnson & Johnson, Johnson & Johnson Services, Inc. and Johnson & Johnson International have been named as Defendants.
According to the Complaint, the Plaintiff received the DePuy ASR hip implant on their left hip in July 2008. Subsequently, the Plaintiff suffered from various personal injuries, including pain. The suit also alleges that the Plaintiff suffered from economic loss and loss of services as a result of their injuries from the DePuy ASR hip replacement.
The lawsuit claims that the Defendants sold the device even though they were aware of its defects. The suit further alleges that the Plaintiff would not have suffered the implant-related damages if the Defendants had adequately warned consumers about the risks of using the DePuy ASR hip implant.
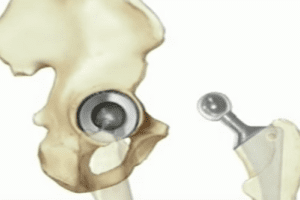
The DePuy ASR hip implant is a metal-on-metal hip implant. This type of hip replacement consists of a metal ball, or stem, rotating within a metal cup. According to the lawsuit, this all-metal design is flawed because it forces the metal surfaces to rub together, causing tiny particles to flake off and get absorbed into the bloodstream. This release of metal ions can allegedly lead to:
In fact, DePuy Orthopaedics recalled the ASR hip implant in August 2010 because the device was failing prematurely in a large number of patients. According to data from the British Orthopaedic Association and the British Hip Society, the failure rate of large diameter metal-on-metal hip implants may be as high as 49 percent in six years.
Most recently, a study published in Orthopedics journal showed that most metal hip revisions occur within two to three years.
Last May, the U.S. Food and Drug Administration (FDA) asked Johnson & Johnson and 20 other manufacturers to conduct post market studies assessing the safety of all-metal hips. The agency also convened a panel of independent experts to analyze the risks and benefits of the implants in June.
At the conclusion of the three-day meeting, panelists said that there is little reason to continue using metal-on-metal hips. They also made recommendations for patients who have the implants, and advised yearly checkups, X-rays, CT scans and other methods for detecting abnormalities.
Parker Waichman LLP continues to offer free legal consultations to victims of DePuy ASR and other metal-on-metal hip implant injuries. If you or a loved one experienced premature failure of your implant or other health problems associated with a recalled DePuy ASR Hip Implant or other metal-on-metal hip replacement device, please contact their office by visiting the firm’s DePuy ASR hip implant injury page at www.yourlawyer.com. Free case evaluations are also available by calling 1-800-YOURLAWYER (1-800-968-7529).
Contact:
Parker Waichman LLP
Gary Falkowitz, Managing Attorney
(800) YOURLAWYER
(800) 968-7529


