
Infusion Pumps Are Being Recalled. The Food and Drug Administration seized infusion pump made by a unit of Cardinal Health, Dublin, Ohio, and the company said it was suspending installation, production, sale and repair of the ‘Infusion Pumps’. Earlier this month, Cardinal’s Alaris Products unit, which makes the pumps, issued an urgent recall notice to customers, […]
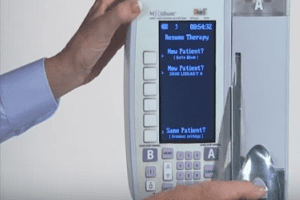
Infusion Pumps Are Being Recalled. The Food and Drug Administration seized infusion pump made by a unit of Cardinal Health, Dublin, Ohio, and the company said it was suspending installation, production, sale and repair of the ‘Infusion Pumps’.
Earlier this month, Cardinal’s Alaris Products unit, which makes the pumps, issued an urgent recall notice to customers, warning that the pumps’ keypads could register numbers twice and lead to overdoses.
Alaris advised healthcare facilities on ways to avoid the error. The company said federal officials seized about 1,300 pumps from a manufacturing plant in San Diego; about 140,000 such pumps have been distributed worldwide.
The personal injury attorneys at Parker Waichman offer free, no-obligation case evaluations. For more information, fill out our online form or call 1-800-YOURLAWYER (1-800-968-7529).


