
Medtronic Diabetes Division Receives an FDA Warning Letter Due to Quality Control Failures DUBLIN, IRELAND – On December 9, 2021, Medtronic PLC received a warning letter from the U.S. Food and Drug Administration (FDA) concerning Medtronic’s diabetes business facility located in Northridge, California. The FDA’s warning letter followed the agency’s inspection of Medtronic’s Northridge facility […]

Medtronic Insulin Pump Lawsuits
DUBLIN, IRELAND – On December 9, 2021, Medtronic PLC received a warning letter from the U.S. Food and Drug Administration (FDA) concerning Medtronic’s diabetes business facility located in Northridge, California. The FDA’s warning letter followed the agency’s inspection of Medtronic’s Northridge facility back in July 2021 and the recall history of Medtronic’s ParadigmTM pumps, MiniMedTM 600 series insulin infusion pump, and a MiniMedTM 508 remote controller device. The agency’s warning letter concentrates on the insufficiencies of certain medical device quality system requirements at Medtronic’s Northridge facility concerning complaint handling, device recalls, reporting of adverse events, risk assessment, and corrective and preventive action.
At this time, Medtronic is not urging patients or healthcare providers to take any specific actions with regards to the FDA’s warning letter delivered to Medtronic on December 9, 2021.
In August 2018, Medtronic issued a Class I Recall of its MiniMed 508 and Paradigm insulin pumps because of a cybersecurity risk involving the device’s remote control.
In November 2019, Medtronic issued a Class I Recall concerning its MiniMed 600 series with an updated retainer ring.
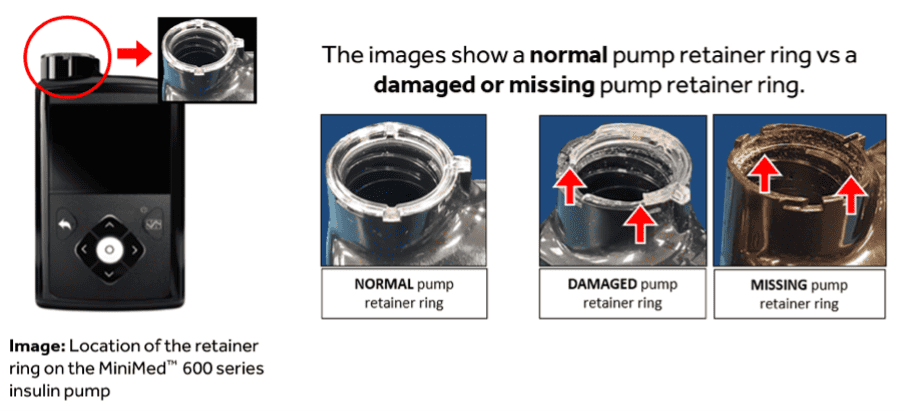
MiniMed™ 600 series Insulin pump
The FDA’s December 9, 2021 letter, the agency discussed:
The FDA is also beginning to enforce many corrective actions correlated with their observations during their July inspection.
Sean Salmon, the President of Medtronic’s Diabetes Business, stated that the company is “committed to fully resolving all observations as effectively and quickly as possible.”
Did you or a loved one suffer harm from a defective insulin device? Parker Waichman LLP helps those who have suffered injuries receive full monetary compensation. Trust your case with our product liability lawsuit lawyers. For a free consultation, contact our law firm today by using our live chat or calling 1-800-YOUR-LAWYER (1-800-968-7529).
MiniMed™ 600 series Image Source: Insulin Pumps Product Description


