
Severe Allergic Reaction On Saphris Warnings. The antipsychotic medication Saphris (asenapine maleate) has been associated with severe allergic reactions, according to a Drug Safety Communication issued by the U.S. Food & Drug Administration (FDA) yesterday. The Saphris drug label has been revised to include information about this risk. The new warnings also inform healthcare professionals that Saphris should not be used […]
Severe Allergic Reaction On Saphris Warnings. The antipsychotic medication Saphris (asenapine maleate) has been associated with severe allergic reactions, according to a Drug Safety Communication issued by the U.S. Food & Drug Administration (FDA) yesterday.
The Saphris drug label has been revised to include information about this risk. The new warnings also inform healthcare professionals that Saphris should not be used in patients with a known hypersensitivity to the drug.
Saphris is an atypical antipsychotic used to treat symptoms of schizophrenia and bipolar disorder. Since its approval in August 2009 up until June 2011, approximately 235,000 prescriptions were dispensed for Saphris and approximately 87,000 patients received a prescription for the drug, according to the FDA.
Between its approval through September 7, 2010, 52 cases that reported Type I hypersensitivity reactions associated with Saphris use were identified in the FDA’s Adverse Events Database.
Some of the cases reported the occurrence of more than one hypersensitivity reaction following Saphris use. Eight cases reported hypersensitivity reactions after just one dose of Saphris.
Of the 52 incidents, 15 reported a resolution of symptoms following Saphris discontinuation, while two of these cases reported a reappearance of symptoms upon reintroduction of Saphris. Nineteen of the cases resulted in hospitalization or emergency room visits, and therapeutic interventions were reported in seven cases.
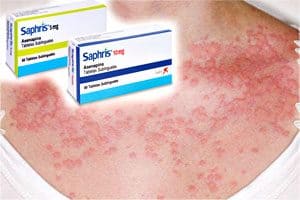
According to the FDA, symptoms of Saphris allergic reactions include: anaphylaxis, angioedema, hypotension, tachycardia, swollen tongue, dyspnea, and wheezing.
According to the FDA, new information regarding the risk of severe allergic reactions has been added to the Contraindications, Warnings and Precautions, Adverse Reactions, and Patient Counseling Information sections of the Saphris drug.
Doctors have been advised to be aware of the risk of hypersensitivity reactions with Saphris and counsel patients who are receiving the drug about how to recognize the signs and symptoms of a serious allergic reaction. Patients should seek emergency medical attention immediately if they develop any signs and symptoms of a serious allergic reaction while taking Saphris.
The personal injury attorneys at Parker Waichman LLP offer free, no-obligation case evaluations. For more information, fill out our online contact form or call 1-800-YOURLAWYER (1-800-968-7529).


