
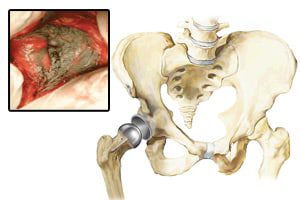
British medical regulators have launched an investigation into metal-on-metal (MoM) hip replacement devices, including the DePuy ASR, over concern that the hips release metal particles into the soft tissue surrounding the joint and these particles enter the bloodstream. Many recipients suffer device failures and injuries and need additional surgery to remove and replace the device. […]
 British medical regulators have launched an investigation into metal-on-metal (MoM) hip replacement devices, including the DePuy ASR, over concern that the hips release metal particles into the soft tissue surrounding the joint and these particles enter the bloodstream. Many recipients suffer device failures and injuries and need additional surgery to remove and replace the device.
British medical regulators have launched an investigation into metal-on-metal (MoM) hip replacement devices, including the DePuy ASR, over concern that the hips release metal particles into the soft tissue surrounding the joint and these particles enter the bloodstream. Many recipients suffer device failures and injuries and need additional surgery to remove and replace the device.
In 2010, the British Medicines and Healthcare products Regulatory Agency (MHRA) recalled the DePuy ASR, a MoM artificial hip, the Buckingham (UK) Advertiser reports. The DePuy was also recalled in the United States.
Both regulatory agencies urged surgeons to inform patients about the DePuy recall, and to schedule DePuy recipients for annual follow-up visits. Patients with symptoms – pain, loosening of the implant, difficulty walking or change in ability to walk – should consider imaging studies and measurements of metal levels in their blood. Removal and replacement of the implant (revision surgery) may be necessary.
The DePuy ASR Acetabular Cup System first came on the market in 2005. The U.S. Food and Drug Administration (FDA) cleared the device through the 510(k) approval process, which allows a manufacturer to obtain approval with very little clinical testing if the manufacturer can prove the device is “substantially similar” to another product already on the market. In 2010, an internal FDA review found numerous flaws with the 510(k) process, and the agency is currently reconsidering the process and is expected to impose testing requirements on all devices prior to approval.
Higher than expected early failure of MoM hips has been a serious concern to both the MHRA and the FDA. While most replacement hips last 15 years or more, researchers told the 2011 annual meeting of the British Hip Society that 49 percent of people who had received DePuy implants needed revision surgery within six years; 12 to 15 percent of those with other metal-on-metal replacement hips needed revision surgery within five years, according to the Buckingham Advertiser. Implant durability is especially important for younger implant recipients, who could live long enough to require a second or even a third hip replacement.


