
MedStream Programmable Infusion Pump and Refill Kits have been recalled; the recall has been deemed a Class I, the U.S. Food and Drug Administration (FDA) just announced. A Class I designation indicates that the action is the most serious type of recall and involves situations in which there is a reasonable probability that use of […]
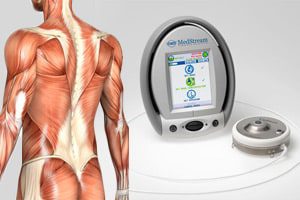 MedStream Programmable Infusion Pump and Refill Kits have been recalled; the recall has been deemed a Class I, the U.S. Food and Drug Administration (FDA) just announced. A Class I designation indicates that the action is the most serious type of recall and involves situations in which there is a reasonable probability that use of the recalled product will cause serious adverse health consequences or death.
MedStream Programmable Infusion Pump and Refill Kits have been recalled; the recall has been deemed a Class I, the U.S. Food and Drug Administration (FDA) just announced. A Class I designation indicates that the action is the most serious type of recall and involves situations in which there is a reasonable probability that use of the recalled product will cause serious adverse health consequences or death.
Codman Neurosciences of Switzerland manufactured the defective devices; Codman & Shurtleff, Inc. issued the recall due to air in the device’s pump reservoir, which may release a higher dosage of a drug than expected. This defect may lead to a drug overdose and may cause patients serious adverse health consequences, including hypotension (low blood pressure), bradycardia (abnormally slow heart rate) loss of consciousness, and/or death.
The MedStream Programmable Infusion Pump is an implanted drug delivery system that is used in the United States for the chronic delivery of Baclofen to treat muscle symptoms. The device is used in Europe, the Middle East, and Africa (EMEA) for the chronic delivery of Morphine or Baclofen. Refill kits are used in the filling and re-filling of the MedStream pump reservoir.
On August 13, 2013, Codman & Shurtleff, Inc. sent a Medical Device Field Safety Notice Letter to its U.S. and international customers describing the affected products and the potential clinical impact. The letter also updated the instructions for use (IFU) pump filling warning and advised customers that the IFU and product training materials had been updated with a warning statement about potential over dosage if air gets inside the pump reservoir, as well as additional clarifications to reinforce the proper filling technique.
Customers were asked to complete the Field Safety Notice Acknowledgement Form and return the completed form to a local Codman Neuro Sales Representative or fax the completed form to 1.508.977.6665.
For questions about the recall, Codman Representatives or Codman Neuro Clinical Support may be reached, toll-free, at 1.800.660.2660. Malfunctions or adverse events associated with the MedStream Programmable Infusion Pumps and refill kits should be reported, toll-free, to 1.866.491.0974 (option 2). Health care providers and consumers may report adverse events or quality problems experienced with the use of medical device or drug to the FDA’s MedWatch Adverse Event Reporting program by completing and submitting the report online at www.fda.gov/medwatch/report.htm or completing the form and submitting it by fax to 1.800-FDA-0178.
The recalled MedStream Programmable Infusion Pumps were manufactured from March 2009 to September 2012 and were distributed from January 8, 2010 to July 19, 2013. A breakdown of the recalled products and related product codes by area is:


