
A newly-published investigation from ProPublica has revealed that several questionable types of medical devices, including DePuy Orthopaedic’s recalled ASR hip implant, managed to make it to market without much scrutiny on the part of the U.S. Food & Drug Administration (FDA). In addition to the metal-on-metal ASR hip implants, Propublica found that transvaginal mesh devices […]
 A newly-published investigation from ProPublica has revealed that several questionable types of medical devices, including DePuy Orthopaedic’s recalled ASR hip implant, managed to make it to market without much scrutiny on the part of the U.S. Food & Drug Administration (FDA). In addition to the metal-on-metal ASR hip implants, Propublica found that transvaginal mesh devices and some heart valve rings were also approved without any premarket clinical trials. Once devices come to market, the FDA’s passive system for reporting adverse events can allow some problems, such as those associated with faulty lead wires used with implantable defibrillators, to remain undetected for years, according to the report.
A newly-published investigation from ProPublica has revealed that several questionable types of medical devices, including DePuy Orthopaedic’s recalled ASR hip implant, managed to make it to market without much scrutiny on the part of the U.S. Food & Drug Administration (FDA). In addition to the metal-on-metal ASR hip implants, Propublica found that transvaginal mesh devices and some heart valve rings were also approved without any premarket clinical trials. Once devices come to market, the FDA’s passive system for reporting adverse events can allow some problems, such as those associated with faulty lead wires used with implantable defibrillators, to remain undetected for years, according to the report.
510(k) Approvals
The FDA’s 510(k) clearance protocols allow manufacturers to apply for FDA approval without conducting clinical trials if they can show a product is “substantially equivalent” to a device already on the market. According to ProPublica, both the FDA and the industry maintain that this fast-track approval process gives patients quicker access to life-saving devices. However, a number of studies have found problems with 510(k) approvals. For example, a review conducted last year by the Institute of Medicine revealed that from 2005 to 2009, three out of four recalled high-risk devices had not been approved with clinical data, but rather had gone through the 510(k) clearance process or had been exempt from review altogether.
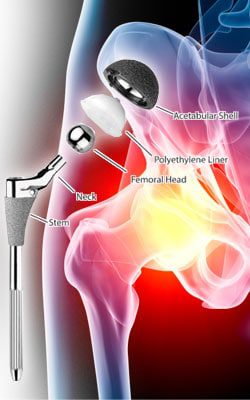
DePuy ASR Hip Implant
As we’ve reported previously, DePuy Orthopaedics, a division of Johnson & Johnson, issued a worldwide recall of the ASR Hip Resurfacing System and ASR Acetabular System in August 2010, after data from the National Joint Registry of England and Wales showed that 1 out of every 8 patients (12%-13%) who had received the devices had to undergo revision surgery within five years of receiving it. By the time the implants were pulled off the market, more than 93,000 people worldwide had received one of the devices.
According to ProPublica, the FDA approved the ASR Acetabular System (the resurfacing system was never sold in the U.S.) under the 510(k) protocols, without any clinical trials to test how it would perform in patient’s bodies. As anyone who reads this blog knows, the recalled DePuy ASR hip implant has caused thousands of patients untold misery, and is now the subject of numerous lawsuits.
The DePuy ASR hip implant was a metal-on-metal device, a class of implants that has come under increased criticism since the August 2010 recall. In March, a large study published in The Lancet showed that there is a 6.2 percent chance patients with all-metal hips will need a replacement within five years, prompting the study’s authors to call for an end to their use. In February, a report in the British Medical Journal, he report warned that hundreds of thousands of people around the world may have been exposed to dangerously high levels of toxic and potentially cancer-causing metals from failing metal-on-metal hip implants. The British Medical Journal also revealed that metal-on-metal hip implant manufacturers were aware of mounting evidence linking the devices to serious, long-term health consequences, but for years failed to warn the public about these dangers.
Like the DePuy ASR device, most metal-on-metal hip implants sold in the U.S. were approved by the FDA via the 510(k) protocols, without the benefit of human testing. Now the agency is playing catch up, and last May directed 21 companies that market all-metal hip replacements, including DePuy, to conduct post-market studies of their products to determine if they were shedding dangerous amounts of metallic debris in patients. Just last month, the FDA announced that its Orthopaedic and Rehabilitation Devices Panel will meet over June 27 and 28 to discuss the risks and benefits of metal-on-metal hip systems, as well as make potential patient and practitioner recommendations for their use.
Transvaginal Mesh
According to ProPublica, numerous surgical mesh products were approved for the transvaginal repair of stress urinary incontinence and pelvic organ prolapse under the FDA’s 510(K) protocols beginning in the late 1990s. In 1999, Boston Scientific recalled its ProteGen Sling, after numerous complaints of pain, infections, and injuries. Today, Johnson & Johnson in embroiled in numerous transvaginal mesh lawsuits involving a product that was approved via a 510(K) clearance because of its similarity to the defective ProteGen sling.
The FDA has been conducting a safety review of transvaginal mesh since 2008. This past July, the agency said in a Safety Communication that it had received 2,874 new reports of complications associated with transvaginal surgical mesh products made by Johnson & Johnson and other companies from January 2008 through December 2010. Of these, 1,503 reports were associated with POP repairs and 1,371 associated with SUI repairs. Injuries attributed to the use of transvaginal mesh devices include
According to the FDA, even when women undergo surgery, sometimes multiple surgeries, to have defective mesh removed, complications continue because it is almost always impossible to completely remove the device. The FDA is now considering rescinding 510(k) approvals for transvaginal mesh devices used in POP repair, and has asked the makers of such devices to conduct safety studies of the products.
Heart Valve Rings
According to ProPublica, annuloplasty rings, circular devices used to repair faulty heart valves, were originally classed high-risk, and not eligible for 510(k) clearance. But in 1997, the industry petitioned the FDA brought to move the heart valve to lower risk class, and the agency complied. Since then, annuloplasty rings have been classed in the same category as hearing aids.
One such device, Edwards Life Sciences’ Myxo Ring, was brought to market in 2006 without even the benefit of a 510(k) approval. According to ProPublica, manufacturers with a cleared device on the market can make changes or modify a product without submitting an entire new application to the FDA, and apparently that’s what happened in the case of the Myxo Ring. However, in 2009, in response to concerns about the way it was brought to market, Edwards recalled the Myxo Ring and applied for FDA approval. The approval was granted in 2009, and the device was relaunched under a new name. While the FDA said Edwards Lifesciences should have sought clearance for the device in 2006, the agency asserted that the company made an “honest attempt” to interpret the regulations, ProPublica said.
Implantable Defibrillator Leads
According to ProPublica, the FDA’s ability to track injuries caused by faulty devices may have allowed problems with implantable defibrillator lead wires – the conductors that connect a defibrillator to a patients heart and deliver life-saving shocks to the organ – to go unchecked for years. Most recently, it was learned that that electrodes inside St. Jude Medical Inc.’s Riata lead could erode out of their lining, potentially causing a short circuit. St. Jude actually warned doctors about the problem and stopped selling the Riata leads in 2010, and officially recalled them last December. Last month, a study published in the journal HeartRhythm found that another type of fault in the Riata lead been responsible for at least 20 deaths.
According to ProPublica, critics point to the Riata lead as an example of FDA failure to adequately monitor devices once they go to market. The agency relies on voluntary reports to track a device’s performance, which critics say allows the types of issues seen with the Riata to go undetected for years.


