
The Food And Drug Administration (FDA) issued a safety alert warning that the complex design of the ERCP endoscope may impede effective cleaning and sterilizing of the reusable device. There are reports of multidrug-resistant bacterial infections in patients who have undergone ERCP (endoscopic retrograde cholangiopancreatography) with reprocessed duodenoscopes, even when the reprocessing instructions are followed […]
Endoscopy Device May Spread Bacterial Infections
The Food And Drug Administration (FDA) issued a safety alert warning that the complex design of the ERCP endoscope may impede effective cleaning and sterilizing of the reusable device.
There are reports of multidrug-resistant bacterial infections in patients who have undergone ERCP (endoscopic retrograde cholangiopancreatography) with reprocessed duodenoscopes, even when the reprocessing instructions are followed correctly. From January 2013 through December 2014, the FDA received 75 medical device reports for approximately 135 patients relating to possible microbial transmission from reprocessed duodenoscopes. The New York Times reports that the FDA alert came a day after California officials reported that seven patients became ill and two had died from what officials said were improperly sterilized endoscopes at Ronald Reagan UCLA Medical Center.
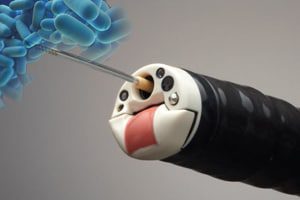
Duodenoscopes, flexible, lighted tubes that are threaded through the mouth, throat, stomach, and into the top of the small intestine (the duodenum), the FDA explains. A hollow channel allows the injection of contrast dye or the insertion of other instruments to obtain tissue samples for biopsy or to treat certain abnormalities. About 500,000 such procedures are performed annually in the United States.
The FDA says meticulously cleaning duodenoscopes prior to high-level disinfection should reduce the risk of transmitting infection, but may not entirely eliminate it. The agency is closely monitoring the association between reprocessed duodenoscopes and the transmission of infectious agents, including multidrug-resistant bacterial infections caused by carbapenem-resistant Enterobacteriaceae (CRE) such as Klebsiella species and Escherichia coli. The FDA reports that it is working with other government agencies, including the CDC, and with manufacturers to identify the causes for transmission of infectious agents and develop solutions to minimize patient exposure.


