
The U.S. Food and Drug Administration (FDA), the U.S. Centers for Disease Control and Prevention (CDC), and the Hawaii Department of Health (DOH) are looking into mounting reports of acute, non-viral hepatitis in Hawaii that may be tied to the supplement, OxyElite Pro, the FDA announced. According to the Hawaii DOH, 24 illnesses have been linked […]
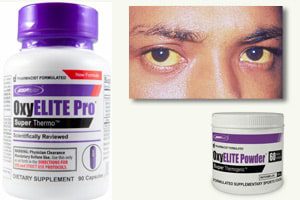 The U.S. Food and Drug Administration (FDA), the U.S. Centers for Disease Control and Prevention (CDC), and the Hawaii Department of Health (DOH) are looking into mounting reports of acute, non-viral hepatitis in Hawaii that may be tied to the supplement, OxyElite Pro, the FDA announced.
The U.S. Food and Drug Administration (FDA), the U.S. Centers for Disease Control and Prevention (CDC), and the Hawaii Department of Health (DOH) are looking into mounting reports of acute, non-viral hepatitis in Hawaii that may be tied to the supplement, OxyElite Pro, the FDA announced.
According to the Hawaii DOH, 24 illnesses have been linked to OxyElite Pro, which is distributed by USPlabs LLC of Dallas, Texas. The supplement is sold nationwide through a number of distribution channels, including the Internet and retail stores that sell dietary supplements.
In Hawaii, a total of 29 cases of acute non-viral hepatitis have been reported. Eleven of these cases involved hospitalization for acute hepatitis. Two people underwent liver transplantation and one person has died. The CDC is also reviewing other liver injury cases nationwide that may be related. Hepatitis, regardless of the type of virus, typically causes fever, fatigue, loss of appetite, nausea, vomiting, abdominal pain, dark urine, clay or gray-colored bowel movements, joint pain, yellow eyes, and jaundice.
The Hawaii DOH and the CDC are conducting an epidemiological investigation and, as part of its related probe, the FDA is reviewing the medical records and histories of those patients identified by the Hawaii DOH, the product samples collected from some of these patients, the facilities involved with product manufacturing, and records concerning production and product distribution. USPlabs LLC advised the FDA that it believes counterfeit versions of OxyElite Pro are being marketed in the U.S. and have been on the U.S. market for some time. Because of this, the FDA is conducting an investigating to determine if counterfeit products are associated with the illnesses.
As of her last report, Dr. Sarah Park, Hawaii state epidemiologist said that all of the cases have involved young to middle-aged adults who suddenly became ill and required hospitalization for various phases of liver failure and, “Who have nothing in common among them except for the fact that they all took a dietary supplement, a nutritional supplement for the purpose of weight loss or muscle-building,” she told KHON.com.
The common finding is that those who have fallen ill is that all have all used a dietary or nutritional supplement for weight loss and/or muscle gain over the past six months, with cases reported in nearly every county in Hawaii. The cases have, to date, involved patients with no infectious causes, not history of high-risk social activities, and no identified commonly expected risk factors for liver failure, according to the Star Advisor.
“OxyELITE Pro” is described on the firm’s website as an update to the former “OxyELITE Pro Super Thermogenic,” and that the new, re-named product has a purple top and is sometimes referred to as the “Purple Top OxyELITE Pro.” The ingredients, according to the product label, include: Bauhinia Purpurea L. (Leaf and Pod) Extract, Aegeline, Norcolaurine HCI, Hemerocallis Fulva (Flower) Concentrated Extract, and Yohimbe (Pausinystalia Hohimbe) (Bark) Extract (AlphaShred™). The original version contained Dimethylamylamine HCl (DMAA), which has since been removed. The new version contains additional caffeine, as well as Noroclaurine HCl, which the website indicated is also known a higenamine, and which goes under the trade name Norcoline™. The website described this ingredient by indicating, “It’s a VERY safe, yet effective, fat targeting agent. Great for focus too!”
The FDA is advising consumers to cease taking any dietary supplement product that is labeled as OxyElite Pro while the investigation continues. The FDA also recommends that consumers who believe they have been harmed by using a dietary supplement contact their health care practitioner.


