
Device maker HeartWare has issued updated information on the multiple recalls for its Ventricular Assist Device (HVAD). The recalls were the result of five different types of complaints from users and doctors. The update provides information on proper performance and safe use of the device to prevent the potential patient injuries, according to a company […]
HeartWare to Recall Ventricular Assist System
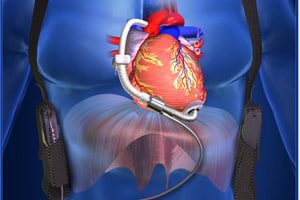
Device maker HeartWare has issued updated information on the multiple recalls for its Ventricular Assist Device (HVAD).
The recalls were the result of five different types of complaints from users and doctors. The update provides information on proper performance and safe use of the device to prevent the potential patient injuries, according to a company news release.
The HVAD helps deliver blood from the heart to the rest of the body. The device is used in patients with end-stage left ventricular heart failure who are waiting for a heart transplant. The system includes a pump implanted in the space near the heart and a controller that controls the speed and function of the pump.
The recall covers HeartWare Ventricular Assist Devices, product codes 1101 and 1103. The 1,763 recalled pumps were manufactured and distributed from January 2008 to March 2015. The Food and Drug Administration (FDA) has classified this a Class I recall, the most serious category, reserved for situations where there is a reasonable probability that use of the product will cause serious adverse health consequences or death.
In a letter to doctors and patients, HeartWare called attention to the following points:
Health care providers with questions should contact their HeartWare representative or HeartWare’s 24-hour Clinical Support at 1-888-494-6365, or email FSCA@heartware.com.
The FDA encourages health care professionals and patients to report adverse events or side effects with the HeartWare system to the MedWatch Safety Information and Adverse Event Reporting Program.
More battery-related cases:


