
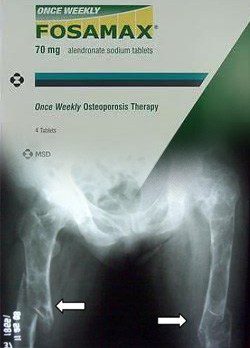
Yet another woman has filed a lawsuit claiming that Merck’s Fosamax (alendronate) caused fractures to her legs. In the Maryland woman’s case, she suffered double thigh fractures. According to the lawsuit, Fosamax and other bisphosphonates can increase risks for atypical femur fractures. She is being represented by national law firm, Parker Waichman LLP. The complaint […]
Woman Claims Fosamax Caused Fractures in Both Thighs
Yet another woman has filed a lawsuit claiming that Merck’s Fosamax (alendronate) caused fractures to her legs. In the Maryland woman’s case, she suffered double thigh fractures. According to the lawsuit, Fosamax and other bisphosphonates can increase risks for atypical femur fractures. She is being represented by national law firm, Parker Waichman LLP.
The complaint indicates that the woman began taking Fosamax around May 2002 and that during the time she was treated with the osteoporosis drug, she suffered two femur fractures. The most recent injury took place in July 2009 when she experienced a break in her left thighbone. The lawsuit alleges that the woman’s injuries are a direct result of using Fosamax for a number of years, and claims severe mental and physical pain and suffering, permanent injuries, emotional distress, economic loss due to medical expenses, and living related expenses due to a new lifestyle. The lawsuit also holds Merck responsible for failing to warn users about Fosamax’s risks and states that sufficient evidence is available to suggest that Fosamax can make bones more brittle and vulnerable to fractures.
Fosamax is approved to treat conditions such as osteoporosis in postmenopausal women and is part of the drug class, bisphosphonate, which is a category that also includes Actonel and Boniva. In October 2010, the U.S. Food and Drug Administration (FDA) updated the label on bisphosphonate drugs to warn about the risk of atypical fractures of the thigh—known as subtrochanteric and diaphyseal femur fractures.
The FDA has also recently questioned the value of using Fosamax and other bisphosphonates as a long-term therapy in women. In May, the agency published a review in the New England Journal of Medicine after it reviewed data collected from over 2,300 postmenopausal women; overall, the research suggested that there is little reason to use the drugs for longer than five years.
Another study, published in the Archives of Internal Medicine, compared patients with classic femur fractures versus those with atypical femur fractures. Among the atypical fracture group, 82 percent were identified as having taken bisphosphonates.
This is not the first lawsuit concerning Fosamax and not the first lawsuit filed on behalf of a patient by Parker Waichman LLP. In fact, the national law firm recently filed several other, similar, lawsuits on behalf of women who took Fosamax and subsequently suffered fractures. The lawsuits name Merck Sharp & Dohme Corp., Merck & Co., Inc., and other potential manufacturers as defendants.
Meanwhile, another recent study of adverse event reports involving Fosamax and other bisphosphonates found that the risk of esophageal cancer associated with these drugs may be higher than first thought. Last summer, the FDA issued a Drug Safety Communication to update the public on its ongoing review of the possible association between oral bisphosphonate drugs and an increased risk of esophageal cancer. At that time, the agency noted that the two studies it reviewed had reached conflicting conclusions and, while the FDA said it believed the benefits of bisphosphonates continued to outweigh their risks, it acknowledged that further study of the issue was needed. Its safety review is ongoing.


