
A man in Louisiana has filed suit against Medtronic Inc. over injuries allegedly cause by off-label use of its Infuse Bone Graft product. The plaintiff, who received Infuse during transforaminal spinal lumbar fusion surgery, claims that the bone growth protein caused unwanted bone growth along his spine, worsening the back pain the surgery was supposed […]
 A man in Louisiana has filed suit against Medtronic Inc. over injuries allegedly cause by off-label use of its Infuse Bone Graft product. The plaintiff, who received Infuse during transforaminal spinal lumbar fusion surgery, claims that the bone growth protein caused unwanted bone growth along his spine, worsening the back pain the surgery was supposed to treat.
A man in Louisiana has filed suit against Medtronic Inc. over injuries allegedly cause by off-label use of its Infuse Bone Graft product. The plaintiff, who received Infuse during transforaminal spinal lumbar fusion surgery, claims that the bone growth protein caused unwanted bone growth along his spine, worsening the back pain the surgery was supposed to treat.
As we’ve reported previously, Medtronic’s Infuse product is only approved for use in single-level anterior lumbar fusion. As such, the procedure performed on the Louisiana plaintiff constitutes an off-label use of the product.
According to the complaint, since his Infuse surgery, Plaintiff Martin Gavin has required epidural spinal injections, physical therapy and the need for additional surgery to implant a transcutaneous electrical nerve stimulator (TENS) unit to help lessen his pain. The lawsuit further alleges that Gavin was led to believe his back pain following his initial surgery in 2011 was the result of the underlying medical condition that necessitated the first procedure. In early 2012, however, he learned that Medtronic Infuse has been associated with unwanted bone growth, which can lead to nerve impingement.
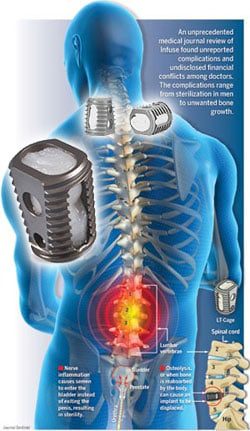
Medtronic Infuse Side Effects
Infuse Bone Graft contains recombinant human Bone Morphogenetic Protein (rhBMP-2), a protein released naturally by the body that promotes bone growth. In 2008, the U.S. Food & Drug Administration (FDA) warned that Infuse and similar products had caused serious and potentially life-threatening problems when they were used off-label in cervical spine (neck) surgeries. Despite such dangers, it is believed that 85% of the procedures using Infuse are off-label.
Last summer, an entire issue of The Spine Journal dedicated to rhBMP-2 products raised serious questions about the validity of the research that was used to gain FDA approval of Infuse. One of the articles published in the journal asserted that Medtronic-sponsored clinical trials had downplayed serious Infuse complications, including:
According to that report, the above Medtronic Infuse side effects occurred in 10 to 50 percent of patients who got the product in 13 company-funded clinical trials between 2000 and 2010.
This past fall, concerns about Medtronic Infuse product deepened when a prominent spine researcher revealed that Infuse may pose a higher cancer risk than first thought. According to Dr. Eugene Carragee, a Stanford University School of Medicine professor and editor of the Spine Journal, research he conducted indicated that patients who underwent procedures using Infuse more than doubled their cancer risk within one year of use. The cancer risk grew to almost five-fold after three years, he said.
Medtronic Infuse Investigations
While doctors can use FDA-approved medical products in anyway they see fit, manufacturers are legally prohibited from promoting off-label use. In 2008, after the FDA warned about complications caused by the off-label use of products like Infuse, the U.S. Department of Justice began investigating the way Infuse had been marketed by Medtronic. The U.S. Senate Finance Committee and the California attorney general are also investigating Medtronic over its marketing of the bone growth product.


