
Medtronic Sprint Fidelis leads are vulnerable to fracture following ICD switch, says a new study published in the June issue of the journal Heart Rhythm. An implantable cardioverter defibrillator (ICD) switch is a fairly common procedure, but the procedure can significantly raise failure risks for the Sprint Fidelis defibrillator leads, a medical device known for […]
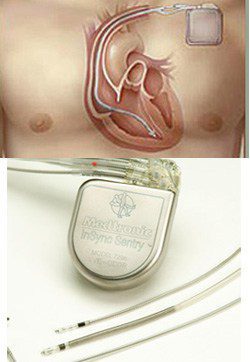 Medtronic Sprint Fidelis leads are vulnerable to fracture following ICD switch, says a new study published in the June issue of the journal Heart Rhythm.
Medtronic Sprint Fidelis leads are vulnerable to fracture following ICD switch, says a new study published in the June issue of the journal Heart Rhythm.
An implantable cardioverter defibrillator (ICD) switch is a fairly common procedure, but the procedure can significantly raise failure risks for the Sprint Fidelis defibrillator leads, a medical device known for it high fracture rates, said Mass Device. The researchers discovered that 1 in 5 Fidelis leads failed following an ICD swap and most failed within the first three months, pointing to the need to replace the lead during ICD swap.
“The replacement procedure itself seems to be the cause, since most of the failures occur within 3 months of the procedure,” the study team wrote, said Mass Device. The researchers observed 72 patients with functioning Fidelis leads who underwent an ICD swap with no lead replacement and found that the Fidelis leads failed in 15 patients after the swap; 60% of these failed within three months. According to Mass Device, the swapped patient failure rate was 20.8% versus 2.5% for patients who did not recently undergo a swap.
A lead is a wire that connects an implantable defibrillator to the heart. If a lead breaks, the defibrillator can emit a massive and painful shock. In the worse case, the fractured lead can prevent a defibrillator from sending a necessary, lifesaving shock to the heart. The Sprint Fidelis lead was recalled in October 2007 after it was determined to have a higher-than-normal fracture, or breakage, rate. Sprint Fidelis lead fractures have been implicated in at least 13 deaths.
As we’ve mentioned, when the Sprint Fidelis was recalled, 268,000 defective Sprint Fidelis leads had been implanted worldwide, and about 235,000 people still had these leads in their chests. At the time of the recall, Sprint Fidelis recipients were advised to leave the leads in place if they were functioning properly, as surgical removal of the tiny wires can lead to serious complications. In fact, it is believed that 4 of the 13 deaths were linked to removal attempts.
According to Mass Device, the wires have been implicated in over 100 deaths; however, Medtronic maintains that its Fidelis leads were only “possible or likely contributing factor” in only 13 of the deaths. Now, said Mass Device, the Sprint Fidelis lead is still active in about 100,000 of the originally implanted patients, according to the study.
Although Medtronic claims that, “Sprint Fidelis performance after device change-out is similar to lead performance without device change-out,” the researchers warn that the supporting data published on this statement was limited. “There are no clinical parameters that predict lead failure after ICD exchange, suggesting that all Fidelis patients should be considered at risk after a generator replacement,” said the researchers, wrote Mass Device.
Meanwhile, said Mass Device, earlier in 2012, another study warned that Fidelis failure rates were rising and some physicians were choosing to replace the faulty leads before they began to fail. “The rate of Fidelis failure continues to increase over time, with failures approaching 17% at 5 years,” the researchers wrote in the January issue of Circulation. “Our data suggests that Fidelis replacement should be strongly considered, at the time of generator replacement,” the researchers added.
We recently wrote that Medtronic agreed to pay $268 million to settle thousands of lawsuits involving its defective Sprint Fidelis defibrillator leads. Since the announcement of the Sprint Fidelis recall, more than 8,000 product liability lawsuits have been filed against the company. As per the new settlement, Medtronic will not be admitting any liability, nor will plaintiffs’ attorneys be admitting that the device maker has a valid defense.


