
Yet another study is adding to concerns about the potential dangers associated with metal-on-metal hip implants. The study, published in the Journal of Bone and Joint Surgery, found that large diameter metal-on-metal total hip replacement may be associated with a substantially higher incidence of pseudotumor formation. The prospective cohort study, conducted by researchers at Isala […]
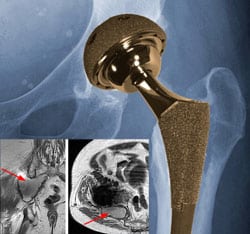 Yet another study is adding to concerns about the potential dangers associated with metal-on-metal hip implants. The study, published in the Journal of Bone and Joint Surgery, found that large diameter metal-on-metal total hip replacement may be associated with a substantially higher incidence of pseudotumor formation.
Yet another study is adding to concerns about the potential dangers associated with metal-on-metal hip implants. The study, published in the Journal of Bone and Joint Surgery, found that large diameter metal-on-metal total hip replacement may be associated with a substantially higher incidence of pseudotumor formation.
The prospective cohort study, conducted by researchers at Isala Klinieken hospital in The Netherlands, looked at a total of 119 patients who underwent 120 metal-on-metal total hip replacements with large-diameter femoral heads between January 2005 and November 2007. The research team obtained outcome scores, serum metal ion levels, radiographs and CT scans for all of the patients. Patients with symptoms or an identified pseudotumor were then offered MRI and an ultrasound-guided biopsy.
Out of 108 patients (109 hips) eligible for evaluation by CT scan at a mean follow-up of 3.6 years (2.5 to 4.5); 42 patients (39%) were diagnosed with a pseudotumor. The hips of 13 patients (12%) were revised to a polyethylene acetabular component with small diameter metal head. Patients with elevated serum metal ion levels had a four times increased risk of developing a pseudotumor.
According to the study authors, the term “pseudotumor” is used to describe a variety of non-neoplastic noninfective lesions in various locations. The term is currently used in arthroplasty of the hip to denote a peri-articular mass caused by an immunological delayed hypersensitivity response to metal particles, characterized by a lymphocyte-dominated histological pattern. Symptoms of a pseudotumor in a metal-on-metal hip implant patient depend on its size and location, and may include:
Because of the high incidence of pseudotumors seen among patients with large-diameter metal-on-metal total hip implant devices, the authors of the study recommended that patients fitted with such devices should be closely monitored for this potential side effect.
Metal-on-Metal Hip Implant Side Effects
Hip implant devices are made from a variety of materials including a ceramic-coated ball in a ceramic cup, a metal ball in a metal cup, and a metal ball fit into a polyethylene cup. Metal-on-metal total hip replacements were introduced for their purported advantages over conventional devices, such as low rates of wear and increased stability. Use of metal-on-metal hip implants using a large size modular femoral head has become increasingly popular during the last decade, especially in young, mobile patients.
Concerns about all-metal hip implants started to mount in 2010, when DePuy Orthopaedics issued a recall of its ASR hip devices, after it was found that they were failing in about 12 percent of patients within five years. Realistically, a hip implant should last around 10-15 years. .
The U.S. Food & Drug Administration (FDA) began studying all-metal hip implants shortly after the DePuy ASR hip implant recall to determine if the devices are shedding dangerous levels of metal ions into patients’ surrounding tissue and blood streams. A number of recent studies have found that the buildup of metal debris can result in tissue damage, the development of cysts and pseudotumors, premature device failure, the need for revision surgery, and other long-term health problems. An FDA advisory panel is scheduled to take up metal-on-metal hip implants during a two-day meeting scheduled for June 27th and 28th.
Metal-on-Metal Hip Implant Lawsuits
The controversy surrounding metal-on-metal hip implants has sparked thousands of lawsuits against DePuy Orthopaedics and other manufacturers. DePuy ASR hip implant lawsuits have been consolidated in federal court in Ohio. In February, a multidistrict litigation was established in the U.S. District Court for the Northern District of Georgia for lawsuits involving the all-metal Wright Conserve Hip Replacement System. Claims involving a metal-on-metal version of DePuy’s Pinnacle hip implant have been consolidated in a multidistrict litigation in Texas. Several lawsuits are also pending in the U.S. over Biomet metal-on-metal hip implants.
Bellwether trials in the DePuy ASR multidistrict litigation are expected to start early next year.


