
Medical device maker Zimmer, Inc. has initiated a voluntary recall of nearly 12,000 Persona Trabecular Metal Tibial knee replacement devices in response to increased complaints of loosening of the device. The recall covers the Persona Trabecular Metal Tibial Plate/Persona TM Tibia-Prosthesis, Knee, Patello/Femorotibial, Semi-Constrained, Uncemented, Porous, Coated, Polymer/Metal/Polymer, all lots land sizes, left and right. […]
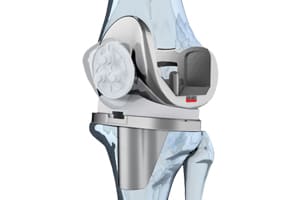
Zimmer Recalls Knee Replacement Device
Medical device maker Zimmer, Inc. has initiated a voluntary recall of nearly 12,000 Persona Trabecular Metal Tibial knee replacement devices in response to increased complaints of loosening of the device.
The recall covers the Persona Trabecular Metal Tibial Plate/Persona TM Tibia-Prosthesis, Knee, Patello/Femorotibial, Semi-Constrained, Uncemented, Porous, Coated, Polymer/Metal/Polymer, all lots land sizes, left and right. Research has shown that radiolucent lines (seen on x-rays), which show the gap between the cement and the device component, can be associated with early failure of the device. Some recipients have needed revision surgery to replace the defective component.
The recalled devices were distributed in Alaska, Alabama, Arizona, California, Colorado, Florida, Illinois, Indiana, Kansas, Massachusetts, Michigan, Minnesota, Missouri, North Carolina, New Jersey, New York, Ohio, Oklahoma, Pennsylvania, Tennessee, Texas, Utah, Virginia, Washington, and Wisconsin, and in Canada, Australia, New Zealand, Korea, Austria, Belgium, Germany, Italy, Luxembourg, Netherlands, South Africa, Sweden, Switzerland, and United Arab Emirates.
The Food and Drug Administration (FDA) has categorized this as a Class II recall. This category is for situations in which “use of, or exposure to, a violative product may cause temporary or medically reversible adverse health consequences or where the probability of serious adverse health consequences is remote,” according to the FDA.
All sizes and lots of the devices are being removed from distribution. Urgent Medical Device Recall notices were mailed to distributors, hospitals, and surgeons last month, asking them to quarantine the affected products until they can be returned to the company. Customers may contact Zimmer at 1-877-946-2761, 8:00 a.m. and 5:00 p.m. EST, with any questions about the recall.
Read more at: Zimmer Ends Relationship With Doctor Following Criticism Of Knee Replacement Component


