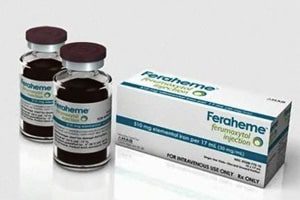
FDA Strengthens Warning Label on Feraheme
The U.S. Food and Drug Administration (FDA) is strengthening an existing warning on the anemia drug Feraheme (ferumoxytol) to reduce the risk of serious, potentially fatal allergic reactions. According to [...]