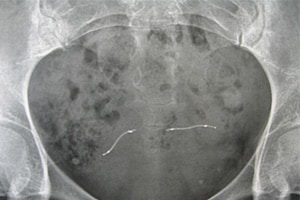
Essure Birth Control Device Complications and Safety Concerns
Essure was potentially dangerous The U.S. Food and Drug Administration (FDA) approved Essure in 2002 and promoted it as a safe and effective method of permanent birth control. It was [...]