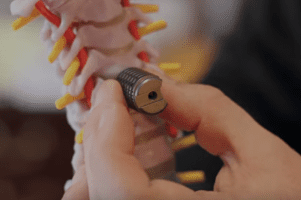
European Regulators Review Medtronic’s InductOs in Light of Contamination Issues
The European Medicines Agency (EMA) will be reviewing Medtronic’s InductOs following reports of contamination problems at a site that manufacturers one of the product’s components. InductOs is an implantable bone [...]