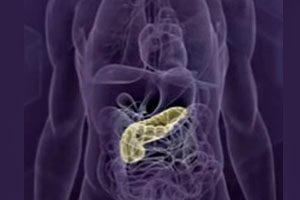
Arkansas Man Claims Byetta Caused Pancreatic Cancer
Complications Developed After Using Byetta. An Arkansas man claims that taking the type 2 diabetes drug Byetta caused him to develop pancreatic cancer. After developing pancreatic cancer and beginning chemotherapy [...]