Marketing Authorization for Medtronic InductOs Device Suspension
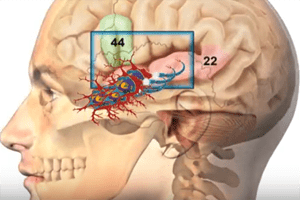
European Medicines Agency Recommend Suspension of Marketing Authorization for Medtronic’s InductOs. The European Medicines Agency (EMA) has recommended the suspension of marketing authorization for Medtronic’s InductOs, an implant to help bone [...]