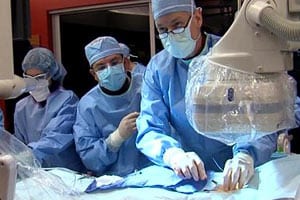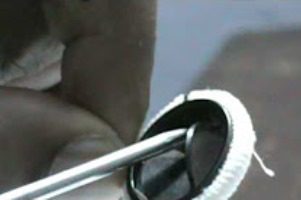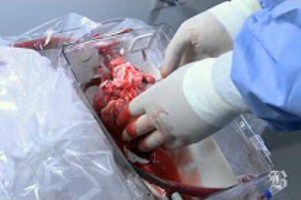
Newly Approved Medtronic Heart Device Component Under Recall
Just weeks after receiving Food and Drug Administration (FDA) approval, Medtronic’s EnVeo R Loading System, used with the CoreValve Evolut R system for transcatheter aortic valve replacement (TAVR), has been [...]