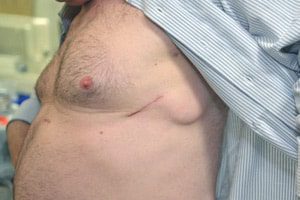
St. Jude Medical Recalls Defibrillator Leads Due to Damaged Insulation
On January 22, 2016, device maker St. Jude Medical announced that a voluntary global field safety action related for the company’s Optisure Dual Coil Defibrillation Leads has now been designated [...]
