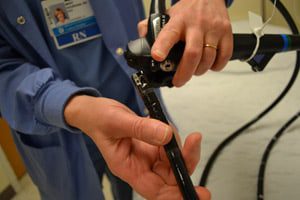
FDA Orders Custom Ultrasonics to Recall AERs Used to Clean Duodenoscopes
The U.S. Food and Drug Administration (FDA) has ordered Custom Ultrasonics to recall all of its automated endoscope reprocessors (AERs), devices used to sterilized special medical scopes such as duodenoscopes. [...]