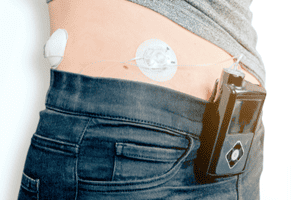
Medtronic MiniMed Insulin Infusion Pump Lawsuits
Medtronic Diabetes Division Receives an FDA Warning Letter Due to Quality Control Failures DUBLIN, IRELAND – On December 9, 2021, Medtronic PLC received a warning letter from the U.S. Food [...]