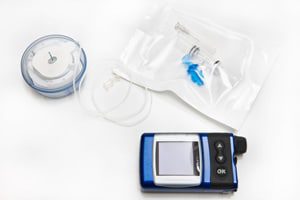
Recall for Medtronic MiniMed 600 Series Insulin Pumps
WASHINGTON, D.C. — Medtronic, the medical device manufacturer, based in Dublin, Ireland, announced an urgent recall of its MiniMed Insulin Pumps in the 600 Series. The pumps reportedly contain a [...]


