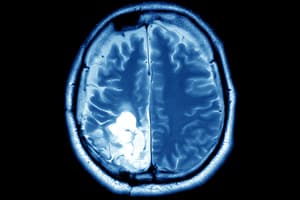
NeuroBlate Injury Lawsuits
The U.S. Food and Drug Administration (FDA) reported that the NeuroBlate system caused significant injuries to patients. Monteris Medical, Inc. manufactured the defective product and had taken corrective measures to [...]

