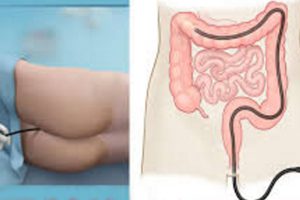
Gastrointestinal Scope Makers Receive FDA Warning Letters
The Food and Drug Administration (FDA) sent warning letters to the three makers of a specialized gastrointestinal scope that has been linked to outbreaks of superbug infections at U.S. hospitals. [...]