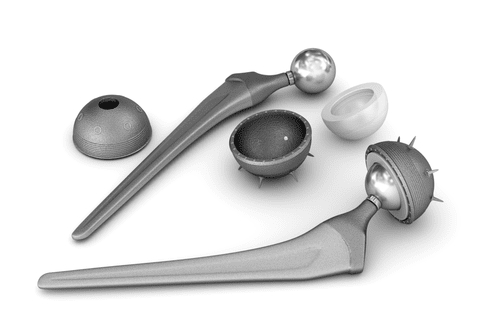
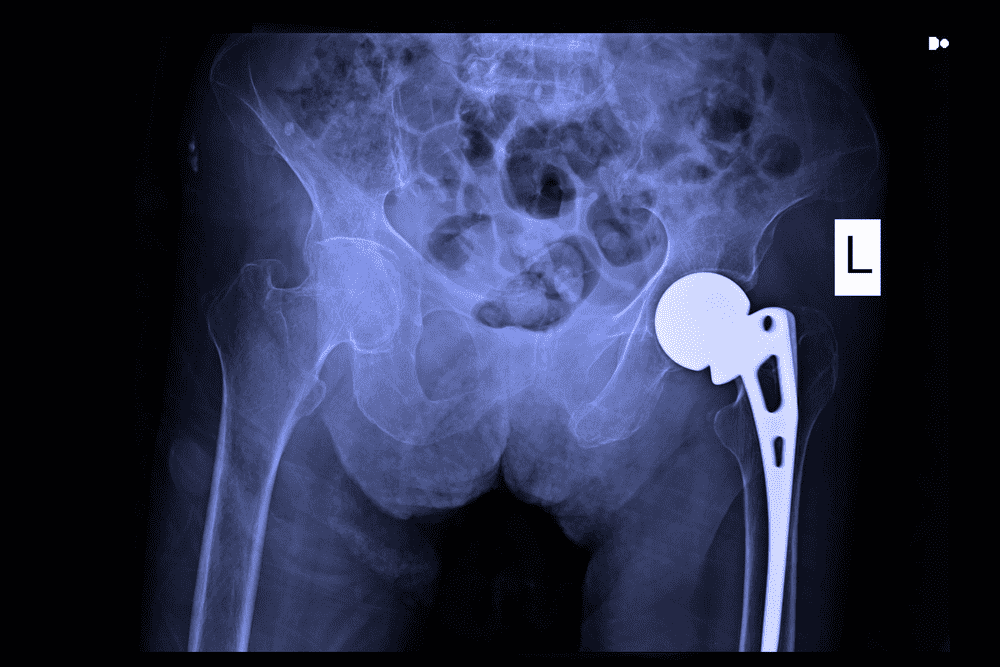
Smith & Nephew Metal-on-Metal Hip Implant Lawsuits
Parker Waichman is offering free legal consultations to individuals seeking to file a Smith & Nephew metal-on-metal hip implant lawsuit. On June 1, 2012, Smith & Nephew recalled a component [...]