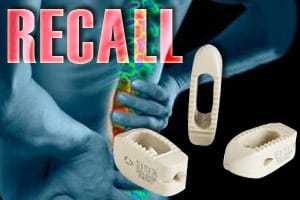
Stryker Oasys Spine Device Recall One in a Stream of Issues
Device Maker Stream of Issues. Undergoing spinal surgery is a frightening and worrisome prospect for patients, and patients agree to these typically complex procedures believing the medical device products being [...]