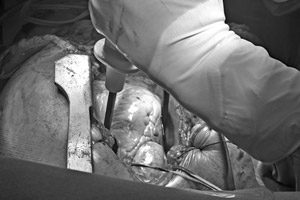
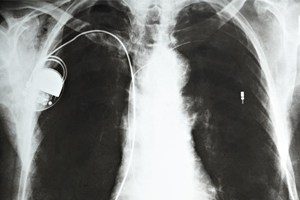
St. Jude Medical Amplatzer Recall Lawsuits
Amplatzer TorqVue FX Delivery System Recall Lawsuits. Parker Waichman LLP is currently investigating lawsuits on behalf of individuals who were injured due to the Amplatzer TorqVue FX Delivery System, [...]