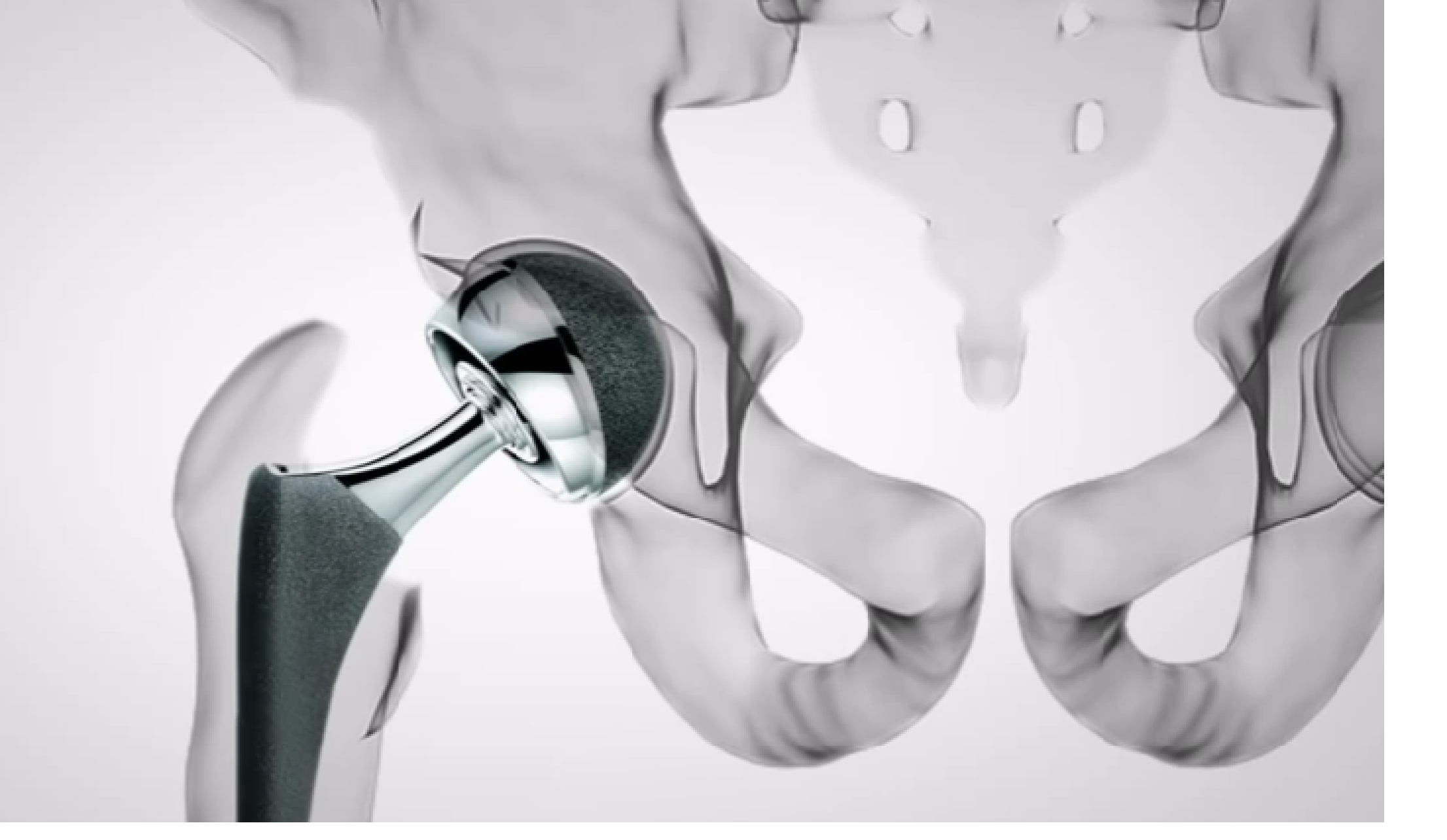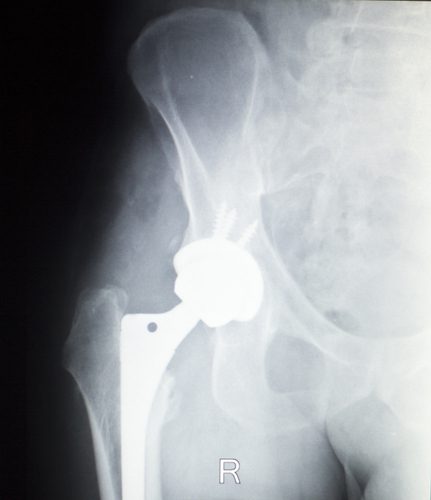
Stryker Rejuvenate, ABG II Hip Implant Settlement Expanded
More Stryker Metal Hip Implant Settlement. More plaintiffs suing over Stryker metal-on-metal hip implants may be eligible for a 2014 settlement involving the recalled Rejuvenate and ABG II modular-neck hip [...]