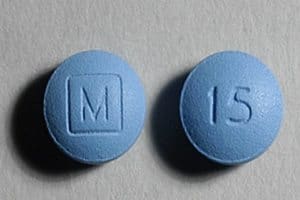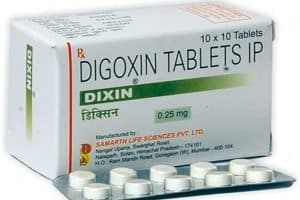
Generic Digoxin Tablets Recalled
Digoxin Tablets Recalled. Potentially defective digoxin tablets have been recalled by generic drug maker Caraco Pharmaceutical Laboratories, Ltd. According to the recall notice, the tablets may differ in size and [...]