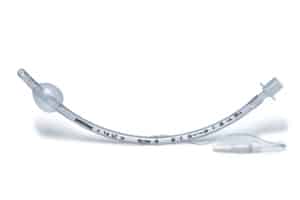
FDA Announces Recall of Teleflex Endotracheal Tubes
The U.S. Food and Drug Administration has announced a recall of certain endotracheal tubes made by the company Teleflex amid concerns that use of the tubes can lead to serious injury [...]