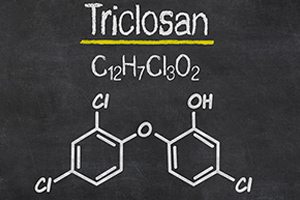
Antibacterial Products May do More Harm
Potential negative effects on consumers’ health To date, there is not sufficient scientific evidence to show that over-the-counter (OTC) antibacterial soaps are better at preventing illness than washing with plain [...]