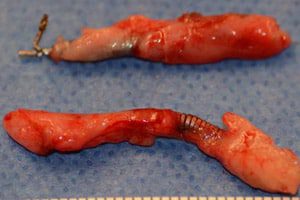
FDA to Announce Essure Review Decision in February
The U.S. Food and Drug Administration (FDA) has said it will announce its decision about what action it will take on Bayer’s permanent birth control device Essure in February 2016. [...]