FDA Says that Drug Makers Must Stop Ranitidine Sales
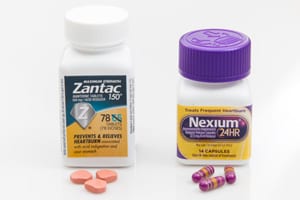
WASHINGTON, D.C. — The U.S. Food and Drug Administration (FDA) told drug makers who sell ranitidine-based medications to stop. Ranitidine is the generic name for the active ingredient in acid-reducing [...]