
Thalidomide Risk of Second Primary Malignancies. The (U.K.) Medicines and Healthcare Regulatory Agency (MHRA) has issued a warning of the risk of developing a second primary malignancy for patients taking thalidomide to treat cancer. Health Canada has issued a similar warning. Thalidomide is licensed in the U.K., Canada, and the United States for treatment […]
Thalidomide Risk of Second Primary Malignancies. The (U.K.) Medicines and Healthcare Regulatory Agency (MHRA) has issued a warning of the risk of developing a second primary malignancy for patients taking
thalidomide to treat cancer. Health Canada has issued a similar warning.
Thalidomide is licensed in the U.K., Canada, and the United States for treatment of previously untreated multiple myeloma in patients age 65 years or older, or those who are ineligible for high-dose chemotherapy.
But the MHRA reports that a statistically significant increase in the risk of second primary malignancies—acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS)—has been observed in an ongoing study of patients receiving combination therapy that includes melphalan, prednisone, and ‘thalidomide’, compared with patients treated with lenalidomide plus dexamethasone.
In an April letter to healthcare professionals, the MHRA said the risk of hematologic second primary malignancies increased over time to approximately 2 percent after two years and 4 percent after three years.
The MHRA advises physicians to weigh the benefits of thalidomide treatment against the risks of developing acute myeloid leukemia and myelodysplastic syndromes, and the agency said patients should be carefully evaluated before treatment and monitored during treatment with standard cancer screenings.
The U.S. Food and Drug Administration (FDA) warns that ‘thalidomide’ should never be taken by pregnant women or women who could become pregnant. Even a single dose taken during pregnancy can cause severe birth defects or death to an unborn baby.
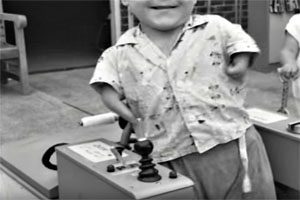
Thalidomide was responsible for severe limb deformities and other birth defects when it was on the market as a sedative in the early1960s.
The personal injury attorneys at Parker Waichman LLP offer free, no-obligation case evaluations. For more information, fill out our online contact form or call 1-800-YOURLAWYER (1-800-968-7529).


