Parker Waichman LLP is investigating cases of retinal detachment complications
MONTGOMERY COUNTY, MD. – July 21, 2017 (immune-oncologynews.com) The FDA is considering adding updated warning information to three cancer drugs to include information about potential eye injuries, according to a news report from immune-oncologynews.com.
Three cancer drugs currently on the market could cause vision loss and retinal detachment:
- Opdivo (nivolumab)
- Yervoy (ipilimumab)
- Keytruda (pembrolizumab)
Opdivo and Yervoy are manufactured by Bristol-Myers Squibb, and Keytruda is manufactured by Merck. All three are approved to treat melanoma, although Yervoy and Keytruda have additional indications, including treatment of non-small cell lung cancer.
Opdivo and Keytruda are both programmed death receptor-1 blocking antibodies. Yervoy is a human cytotoxic T-lymphocyte antigen 4-blocking antibody. All three drugs, however, work to change the immune system’s response to cancer cells by manipulating the body’s T cells. All three drugs interfere with T cell function to allow these cells to attack cancer cells, thereby creating an immune defense against cancer within patients’ bodies.
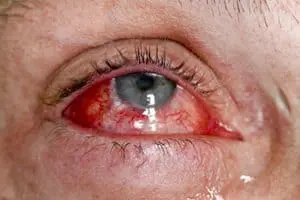
While the drugs have seen success, the post-market information reveals a potential risk associated with all three, including vision loss and retinal detachment. A recently published study detailed a patient who took Yervoy and suffered vision loss in his left eye four months after treatment. The same patient also experienced intermittent vision loss in his right eye five months after treatment. The patient’s injuries were found to be immune-related adverse reactions (IrAR) to the drug.
This patient represents numerous reports of vision injuries associated with the drugs. In early 2017, the U.S. Food and Drug Administration (FDA) released a notice titled, “Potential Signals of Serious Risks/New Safety Information Identified by the FDA Adverse Event Reporting System.” Keytruda, Opdivo, and Yervoy were all on the report with the potential serious risks of “ocular toxicities including vision loss and retinal detachment.” At this time, however, the FDA has taken no further action, and the drug labels remain unchanged.
Currently, all three drugs’ labels contain information about a condition called uveitis, which causes inflammation of the eye. The Yervoy label also currently contains a Black Box warning, which is the strongest possible warning for a drug label, regarding severe and fatal immune-mediated adverse reactions. The warning says these reactions can involve any organ system, with the most common reactions being enterocolitis, hepatitis, dermatitis, neuropathy, and endocrinopathy.
Regarding new information about eye injuries, the FDA is “evaluating the need for regulatory action.” Should the FDA find that adverse event information and subsequent studies warrant additional warnings inside the drug labels, it will direct the two manufacturers to change their labels. The FDA will also communicate the new warning information, and the manufacturers could take the additional step of notifying doctors who prescribe therapy with the three drugs.
In the meantime, the manufacturers will continue monitoring the safety of the drugs, including reports of adverse events, and will update the FDA if they discover additional information related to the risks of eye injuries. Patients who use Opdivo, Yervoy, or Keytruda — or some combination of the three — are encouraged to monitor their own symptoms and talk to their doctors right away if they notice any changes in eyesight. Following the appropriate medical intervention, patients are encouraged to report their experiences to the FDA, the manufacturer(s), or both.
The FDA’s Adverse Event Reporting System is one key way the Administration tracks post-market information on prescription drugs. When numerous instances of one particular adverse event appear in the system, the FDA starts to analyze whether the drug label needs to be updated and whether patients and doctors need to be warned. Additional information regarding the Adverse Event Reporting System can be found at fda.gov.
Contact Parker Waichman LLP for Your Free Retinal Detachment Case Evaluation
If you or your family member has been taking a cancer medication and suffered retinal detachment, contact our firm today for your free consultation by filling out our online form or by calling 1-800-YOURLAWYER (1-800-968-7529).







