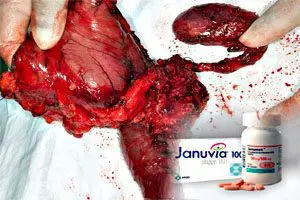
Potential Link Between Januvia and Pancreatic Cancer. Federal health officials are set to launch an investigation into a possible link between the type 2 diabetes drug Januvia (sitagliptin) and a risk of pancreatic cancer.
According to a New York Times report, the U.S. Food and Drug Administration (FDA) is acting on the research of a single person who said he found that lab rats taking Januvia had developed
pancreatic cancer.
Dr. Peter C. Butler, chief of endocrinology at UCLA, was tapped by Merck & Co., the maker of Januvia, to conduct a safety study on its top-selling type 2 diabetes drug. He originally declined the offer but then accepted and made his troublesome discovery.
After finding that lab rats introduced to Januvia had developed pancreatic troubles, the FDA along with the European Medicines Agency said they would develop the study further and investigate the potentially deadly link associated with the drug, according to The New York Times, which also reports that the last time the FDA acted on the work of a single researcher was in the case of another type 2 diabetes drug, Avandia.
Avandia has since been severely restricted from the U.S. market
Avandia has since been severely restricted from the U.S. market and banned outright in countries covered by EMA. Based on our previous reports, Dr. Butler’s assessment on the safety of Jaiabetes Drug Januvia and Pancreatic Cancernuvia is not the first to link the drug to possible pancreatic cancer.
Previous studies have also suggested that diabetics taking Januvia also face a risk of pancreatitis (an inflammation of the pancreas) and thyroid cancer.
Januvia already carries a warning about the risk of pancreatitis, but information from the Mayo Clinic, according to our previous accounts, indicates that pancreatitis and diabetes make a person more likely to develop pancreatic cancer. Pancreatic cancer is often not diagnosed until it reaches its later stages.
The FDA announced in March through a Drug Safety Communication that pancreatic tissue taken from patients who had been on Januvia showed the kinds of cellular changes that often precede pancreatic cancer, according to our previous reports.
Need Legal Help Regarding Januvia and Pancreatic?
The personal injury attorneys at Parker Waichman LLP offer free, no-obligation case evaluations. For more information, fill out our online contact form or call 1-800-YOURLAWYER (1-800-968-7529).
[sc name=”post-footers”]