Fosamax Develop Thigh Fractures on Patients Opening statements were made yesterday in a New Jersey courtroom during a new trial over claims that Merck & Co.’s osteoporosis drug Fosamax makes patients more susceptible to thigh fractures.
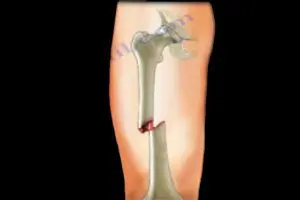
This case is one of over 3,300 pending cases alleging that Merck’s Fosamax caused so-called atypical femur fractures. The plaintiff, Bernadette Glynn, 59, took Fosamax for more than six years before suffering a femur fracture while bending over to pick something up. These so-called atypical femur fractures are often the result of traumatic events, such as high falls, car accidents, or skiing mishaps. Glynn alleges that use of ‘Fosamax’ alone makes patients susceptible to these kinds of injuries.
The U.S. Food and Drug Administration (FDA) approved Fosamax in 1995 to treat and prevent postmenopausal osteoporosis and to treat other bone loss-related issues, including weakened bones following some cancer treatments. In 2010, the FDA updated the safety information on ‘Fosamax’ to warn of the risk of atypical femur fractures. The agency published a review in the New England Journal of Medicine in 2012 suggesting there is little benefit from taking bisphosphonates, the class of drugs that includes ‘Fosamax’, for longer than five years.
Merck violated federal regulations by not warning doctors and patients
Plaintiffs’ counsel Paul Pennock alleged that Merck violated federal regulations by not warning doctors and patients of the problems associated with ‘Fosamax’. “Doing so is not optional—warning ‘Fosamax’ users of the risks is their obligation.” Pennock told the jurors that Merck’s own documents would show that five years before FDA approval, “the company was concerned about femur fractures, yet never warned about these dangers.”
Matthew J. McCauley, senior litigation counsel at Parker Waichman LLP, which has filed approximately 200 femur-fracture cases, said “the jury will see the extent of what Merck knew about the potential damaging side effects related to ‘Fosamax’.”
Need Legal Help Regarding Fosamax Trial?
The personal injury attorneys at Parker Waichman LLP offer free, no-obligation case evaluations. For more information, fill out our online contact form or call 1-800-YOURLAWYER (1-800-968-7529).