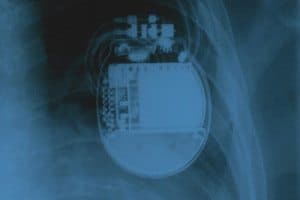
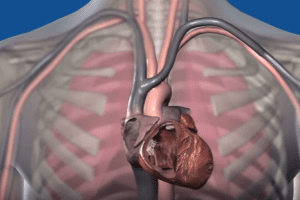
Boston Scientific Emblem S-ICD Defibrillator System Electrode Recall
FDA Issues a Class I Recall of Boston Scientific’s Emblem S-ICDs Due to Reports of Injuries and Deaths FDA – The U.S. Food and Drug Administration has specified a recall of Boston [...]